Cars and flow batteries?
According to IDTechEx, lithium-ion batteries will dominate the automotive scene over the coming ten years and possibly the coming 15 years. During that time we shall see peak lead acid battery the biggest nail in its coffin being when electric vehicles more widely abandon them and even those conventional vehicles modified to stay legal under impending carbon dioxide laws abandon them, relying totally on lithium-ion units.
However, that is not to say that there are no other contenders. IDTechEx have closely assessed the research and see lithium-sulphur as in the lead but with considerable challenges to overcome before it hits prime time such as life and volumetric energy density – being too big though lighter.
Wild card
One wild card is the flow battery. Most people rightly see these as very large units suitable for grid applications such as peak shaving but one or two organisations are thinking the unthinkable about putting them in cars because they are headed for $100 per kWh and size and weight might just fit the bill in due course. Toyota’s advanced research people told us recently that they would not dismiss the possibility.
Nanoflowcell has been exhibiting sports cars it says will use their flow batteries in pure electric form at 48V and achieve 1000 miles pure electric range. Even that voltage is counter to received understanding on design of car powertrains. Pure electric cars are generally headed up towards 800V for light weight and performance. Yes there is a huge move to keeping conventional cars legal under impending pollution laws by making them 48V at least in part but not pure electric vehicles where 48V is typically only seen in very weak duty cycles such as golf cars and postal delivery vehicles.
Flow battery technology is immature. There is everything to play for and although pumping liquids from reservoirs does not sound compact or reliable in a car but who knows?
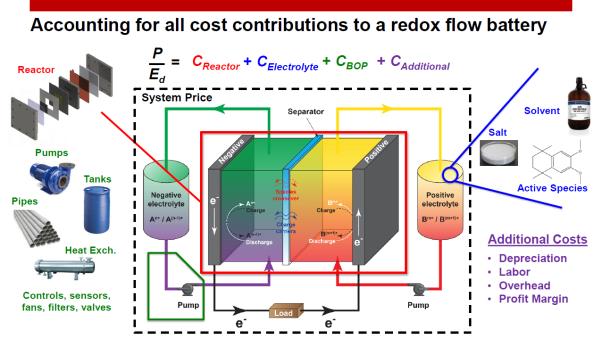
Redox flow batteries typically have a very low energy density, which is roughly one order of magnitude lower than the best Li-ion. The way Nanoflowcell aim at introducing redox flow technology in a car is by making use of a non-rechargeable chemistry. An analogy can be drawn from hearing aid devices, which rely on zinc-air batteries: these batteries can last for much longer than Li-ion, NiMH, or other chemistries, but have to be replaced with new ones once they reach their end of life.
Such a model obviously becomes economically not feasible when we ramp up from microwatt-hours to kilowatt-hours for an EV, but redox flow cells enjoy an added benefit that other battery chemistries lack.
In its simplest configuration, a redox flow battery comprises two electrolyte tanks (the anolyte and the catholyte) that react with each other via redox reactions. Once the energy contained in both the anolyte and the catholyte is completely consumed, these liquids are replaced with new ones, the same way an ICE car replenishes its tank with gasoline, and the car. The rest of the flow battery remains in the car and the spent electrolytes can be hopefully collected, recycled and reused by another customer.
If the combined cost of the electrolytes, of the refuelling infrastructures, and of the recycling process is competitive, Nanoflowcell can be expected to deliver a pure electric car that can be charged within minutes, offer a good driving range, and alternative battery chemistry for the automotive industry.


